Types of Defective Medical Devices
At McIntyre Law, we specialize in helping suffering victims get the compensation they deserve from manufacturers of these types of defective medical devices.
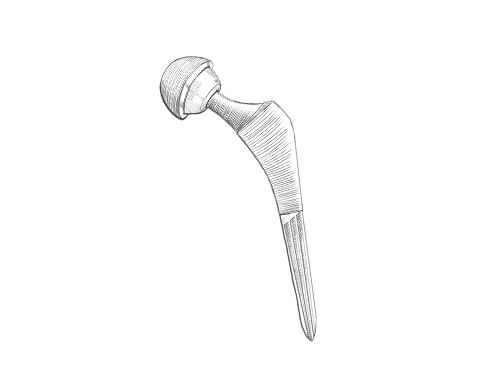
Hip Implants
Metal-on-metal hip implants can fail or spread toxic metal into surrounding tissues, causing pain, swelling, and metal poisoning.
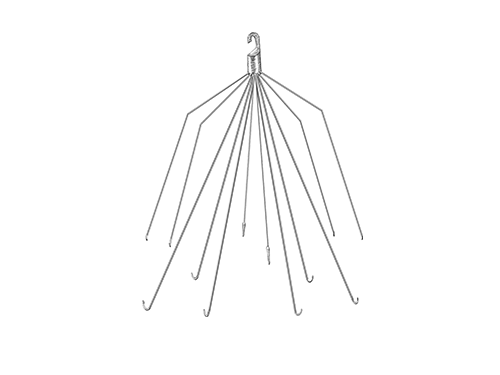
IVC Filters
Many blood clot patients had IVC filters implanted, which can fail, break apart, migrate, or become lodged, causing injury or death.
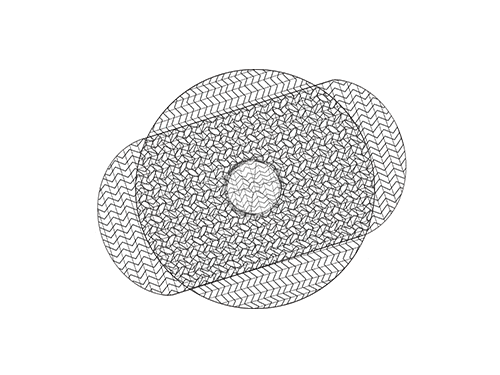
Hernia Mesh
Hernia mesh implants may cause infections, bowel obstruction, and hernia recurrence. Thousands of victims are now coming forward.
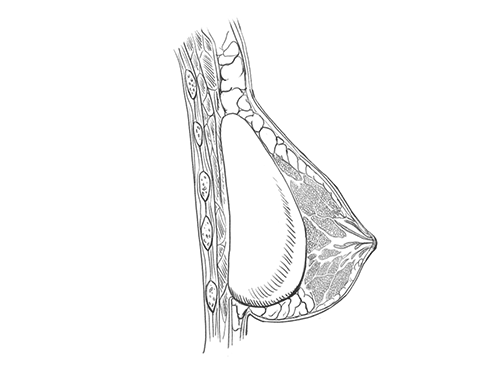
Breast Implants
Defective or leaking breast implants can cause breast pain, infections, numbness, dead skin, and other complications.
Are there currently any class-action medical device recall lawsuits?
In class-action and multi-district litigation lawsuits, dozens or hundreds of cases from around the country are brought under the jurisdiction of a single court. This court could be anywhere in the country—but no matter where you are, McIntyre Law’s experienced attorneys will provide dedicated, caring service. And our founder, Noble McIntyre, is always just a phone call away.
What to Know Before You File a Lawsuit
If you’ve been the victim of faulty or defective medical devices, it’s crucial that you have experienced representation on your side. You’ll need an attorney who understands how a medical device recall works and who will fight for the compensation you deserve.
Don’t go up against the big medical device manufacturers on your own. You will need to acquire medical records, billing records, and file legal papers like petitions and summonses. Our professionals will do most of this work for you. We’ll make sure you have experienced, strategic lawyers by your side to help you get the compensation you deserve.
The Statute of Limitations for Medical Device Recall Lawsuits
Once you suffer harm from a medical device, the clock starts ticking on your right to file a lawsuit. In some states, the statute of limitations is as little as one year. That’s why, if you feel you may be suffering harm from a medical device, you should contact an experienced attorney right away.
What is a contingency fee?
When a lawyer works on a “contingency fee” basis, they’re agreeing to accept a fixed percentage of any monetary rewards from your lawsuit to cover their legal fees. If there are no rewards from the case, you don’t pay the lawyer. We get paid if, and only if, you do.
What compensation can I get from a defective medical device lawsuit?
Courts recognize that suffering victims deserve monetary compensation for both money they have lost—like paying medical bills—and pain they have suffered. Among the types of compensation you could be eligible for are:
- Cash awards
- Reimbursement of current medical expenses
- Subsequent medical procedures
- Lost wages
Working with McIntyre Law
At McIntyre Law, we give personal injury victims experienced, strategic legal representation. We know that’s the best chance someone like you has to get the compensation they deserve. Make sure you have the best possible partner as you seek justice and fairness.
Do you have a case for defective medical device lawsuit?
Anyone suffering from a medical device implant has the right to seek compensation. It doesn’t matter if the device has been recalled, or if a lawsuit is already in process. If you are suffering, contact one of our attorneys. You’ll find out after a short phone call whether your case is likely to result in monetary compensation. Finding out for sure will give you and your loved ones peace of mind.
Meet Our Defective Medical Device Attorneys
Let our experienced medical device lawsuit attorneys fight for the compensation you deserve.
What causes a medical device recall?
Medical device companies have a duty to ensure that products are fully tested and safe for surgical use. They also must fully disclose all risks associated with the use of their products. A patient can’t make an informed decision about surgical options without full and complete disclosure about any medical devices. What if the manufacturer doesn’t have all of the information, either? Sometimes, a medical device recall happens because the company rushed the product to market, rather than fully researching it to make sure it’s safe. Of course, the manufacturers want to maximize their profits, but sometimes that comes at a cost to your health.